Top Tips - Costing a Research Project
Who can help me with my costing?
NIHR Clinical Research Network (CRN) can advise on the feasibility of delivering studies in the NHS and in non-NHS locations. Their AcoRD specialists will provide expert advice on cost attribution and SoECAT completion and authorise your costing prior to grant submission. Contact CRN at an early stage, allowing a minimum of 10 working days to do this: supportmystudy@nihr.ac.uk
Consider these questions:
- Are your projected costs realistic? (Will they adequately cover all identified research activities?)
- Is your research project value for money?
- Are you reimbursing expenses incurred by patients or members of the public involved in your project?
- Will you need to pay for any other assistance or do you have enough staff within your research project?
- Do members of the research team need training and does this have cost implications?
- Have you considered costs for equipment procurement and ongoing maintenance, software licences, travel expenses, office supplies (printing, photocopying, postage, stationery, advertising), costs associated with hiring translators, transcription costs, literature reviews, access to available publications, dissemination costs, etc.?
- Do you have CTU involvement in your project? (This will need to be costed.)
- Have you taken into account the three main types of cost: research costs, NHS support costs and treatment costs in line with AcoRD (see below)?
- Have you allowed sufficient time for the costing to be verified by the host institution’s finance office and the finance office of any collaborating partners? You will need to check the timelines that they work within.
- Do you need to complete a SoECAT (Schedule of Events Cost Attribution Template)? If yes, contact your local CRN to access advice and support from CRN AcoRD Specialists. Allow a minimum of 10 working days for an authorised SoECAT.
Attributing the costs of health and social care Research and Development (AcoRD)
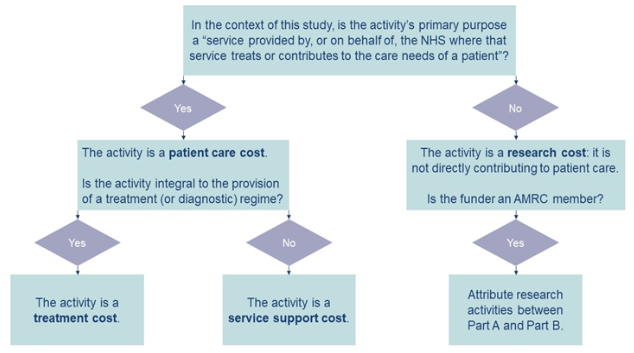
The AcoRD guidance categorises the costs associated with research based on the nature of the activity and whether activities contribute to NHS patient care. Activities are divided into three categories, to which all research and development activities can be attributed:
- NHS treatment costs are patient care costs, which will continue to be incurred if the patient care service in question continues to be provided after the R&D study ends. These costs might be higher or lower than the cost of providing the current standard treatment in the NHS. Where the costs are higher, these are known as Excess Treatment Costs. Excess Treatment Costs are met through the normal commissioning process.
- NHS service support costs are additional patient care costs associated with the research, which will end once the R&D study in question concludes, even if the patient care involved continues to be provided. Examples of such costs are processing patient records to identify NHS patients eligible to participate in the study and obtaining informed consent from participants. These costs are met from the R&D budget of the Department of Health and Social Care and are provided through the NIHR Clinical Research Network (CRN) with the activities carried out by CRN-funded staff.
- Research costs are costs of the R&D itself that end when the research ends. They relate to activities that are being undertaken to answer the research questions. Research costs are split into two parts: Part A which must always be met by the funder and Part B which will be met by the DHSC where the funder is an Association of Medical Research Charities (AMRC) member and NIHR Non-commercial partner (see NIHR website).
-
-
Part B costs might include:
- local study trial co-ordination and management
- data collection needed to answer the questions that the research study is addressing
- regulatory preparation and compliance, including obtaining ethical approval and complying with the Medicine for Human Use (Clinical Trials) Regulations 2004
- time taken by chief and principal investigators (CI and PI) to explain the study to professional colleagues and to understand the research elements of a study.
-
A list of common research activities attributed using AcoRD can be found in Annex A.
Online training resources for AcoRD and SoECAT completion are available via NIHR Learn.
Costings flowchart
Next steps
- Contact your institution’s research support office
- Contact your local NHS Trust R&D office
- Contact CRN https://www.nihr.ac.uk/explore-nihr/support/study-support-service.htm
- Contact Research Support Service for advice on study design: https://www.nihr.ac.uk/rss
Author: Louise Halbert Created: March 2021
