|
|
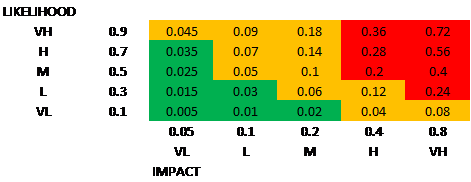
Description
|
|
Technology
|
- Technology not proven
- No recognised clinical need
- Concept not innovative
- All technical requirements not covered by the team
- Risk to the environment (waste production, not fitting net zero NHS)
|
|
Finance
|
- Cost estimates not realistic
- Budget over-runs
|
|
Project Management
|
- Resource issues/incorrect team build
- Loss of key personnel
- Organisational changes
- Uncontrolled changes to scope of project/project scope not set
- Lack of coordination between the project partners
- Project delays
- Incorrect innovation model
- Project time scales not achievable
- Milestones not realistic (go/no-go criteria)
|
|
Public Engagement
|
- Mis-interpretation of facts
- Biased outcomes from group (group structure)
- Inadequate training of participants
- Product is not acceptable to public involvement group
- Stakeholders not available for reviews/testing
|
|
Development
|
- Cannot automate production
- Cannot scale up
- Stability issues
- Incorrect evaluation/validation
- Equipment issues
- Product too complex for the proposed development
- Design issues
- Unclear user interface
- Required performance not met
|
| Software |
- Does not work
- End users reject software
- Poor data for analysis
- Does not fit with NHS (other) systems
- Data storage issues
- Unclear user interface
|
|
Regulatory
|
- Ethics approval delays
- Ethical issues with data sharing with a partner
- Regulatory change
- Changes to Medical Device Regulations
- Regulatory hurdles impact on the commercial uptake of the technology
- System approval, and accreditation issues
- Difficulties or delays in obtaining a Letter of No Objection from the MHRA
|
|
Clinical Study
|
- No clear clinical outcomes
- Clinically ineffective
- The study will not provide enough clinical information to advance the project
- Safety/tolerability issues arise from the clinical study
- Failure to recruit patients
- Lower than predicted number of study participants
- Higher than predicted participant drop-out from the study
- Conflict over results between sites
- Adequate quantity and quality /robustness of clinical data not generated
|
|
IP&Commercialisation
|
- Freedom to Operate issues
- Loss of IP
- Weak IP protection
- Inadequate outcome/impact measures
- Contracts cannot be made with key partners
- Not economically viable to NHS
- Cannot integrate into routine clinical pathway
|
| Market |
- Commercial failure
- Technology change impacts project
- Market changes
- No incentive to change clinical practice
- Inadequate return on investment (ROI)
- Competing solutions
- Market slow to adopt
- Diversion of NHS resources to other urgent needs
- Health economic case and impact not clear
|
|
Adoption&Dissemination
|
- Stakeholders/champions not engaged
- End users reject the product
- Product is not acceptable to NHS (cost, lack of evidence etc)
- Slow adoption/dissemination
- Product not adequately tested before implementation
- Does not fit NHS pathways
|
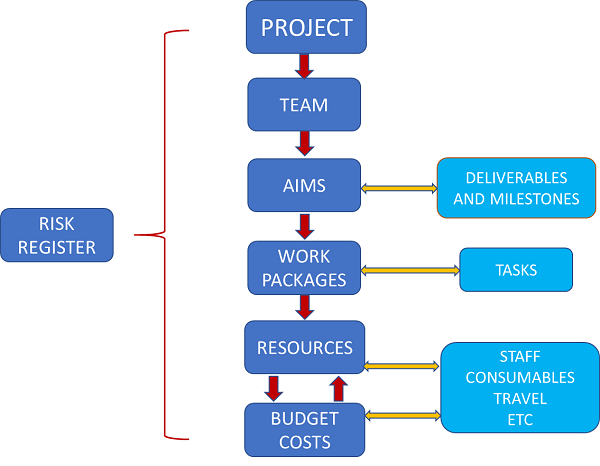

 Your Idea
Your Idea
 Research Planning
Research Planning
 Project Planning
Project Planning
 Impact
Impact